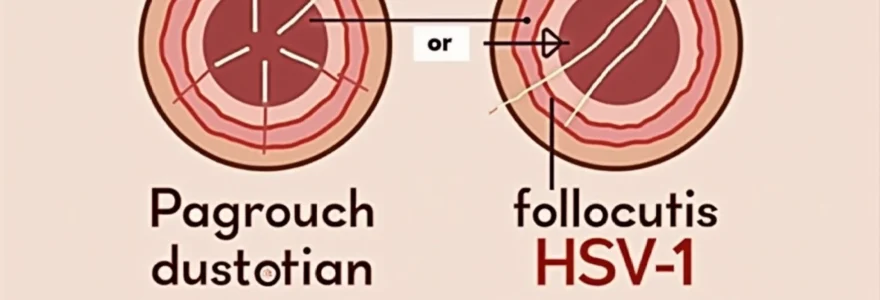
Distinguishing between ingrown hairs and herpes lesions represents one of the most common diagnostic challenges in dermatological practice. Both conditions can manifest as painful, inflamed bumps in similar anatomical regions, particularly around the facial and genital areas. The clinical significance of accurate differentiation cannot be overstated, as these conditions require entirely different therapeutic approaches and have vastly different implications for patient health and quality of life.
The confusion between these two distinct pathological entities often stems from their superficial similarities during initial presentation. However, understanding the underlying pathophysiological mechanisms, recognising specific clinical patterns, and employing appropriate diagnostic methodologies can provide healthcare practitioners and patients with the tools necessary for accurate identification. This comprehensive examination explores the intricate details that separate follicular inflammation from viral infection, offering evidence-based insights for clinical decision-making.
Clinical manifestations of folliculitis barbae and HSV-1 lesions
The clinical presentation of ingrown hairs and herpes lesions exhibits distinct characteristics that, when properly recognised, facilitate accurate diagnosis. Folliculitis barbae , commonly known as razor bumps or ingrown hairs, typically manifests as isolated papulopustular lesions that develop within hair-bearing regions following mechanical trauma from shaving or hair removal procedures.
Papulopustular eruptions in pseudofolliculitis barbae
Pseudofolliculitis barbae presents as inflammatory papules and pustules that develop when curved hair shafts penetrate the skin surface at acute angles. These lesions typically measure 2-5 millimetres in diameter and exhibit a characteristic erythematous base with a central pustular head. The inflammatory response surrounding ingrown hairs often creates a firm, tender nodule that may persist for several days to weeks without appropriate intervention.
The distribution pattern of these lesions follows the contours of hair growth and shaving practices. In males, this predominantly affects the beard area, particularly the neck region where hair growth patterns create optimal conditions for follicular penetration. The lesions typically appear 24-48 hours following shaving activities, correlating directly with the hair growth cycle and mechanical irritation.
Vesicular herpetic lesions and crusting patterns
Herpes simplex virus lesions follow a distinctly different morphological progression that begins with prodromal symptoms including tingling, burning, or localised pain. The initial vesicular stage presents as small, fluid-filled blisters measuring 1-3 millimetres in diameter, often appearing in characteristic clusters or groups. These vesicles contain infectious viral particles and typically rupture within 24-48 hours, creating shallow ulcerations.
The subsequent healing phase involves crust formation as the ulcerated lesions begin to epithelialise. This crusting pattern represents a pathognomonic feature of herpetic infection, distinguishing it from the pustular drainage typical of follicular inflammation. The entire cycle from vesicle formation to complete healing typically spans 7-14 days, depending on individual immune response and treatment intervention.
Anatomical distribution differences in facial regions
The anatomical distribution of lesions provides crucial diagnostic information for differentiating between these conditions. Ingrown hairs demonstrate a clear predilection for hair-bearing areas subjected to regular grooming practices, including the lower face, neck, and jawline in males. The distribution typically follows shaving patterns and may be asymmetrical based on individual grooming habits.
Conversely, herpes simplex lesions exhibit a tendency to recur in consistent anatomical locations, following the distribution of affected sensory nerve pathways. Primary herpetic infections may present with more widespread involvement, whilst recurrent episodes typically localise to specific sites such as the vermillion border of the lips or consistent facial regions. This pattern of recurrence in identical locations represents a key diagnostic feature of viral aetiology.
Temporal progression of inflammatory responses
The temporal evolution of these conditions differs markedly in both onset and resolution patterns. Ingrown hair inflammation develops gradually over several days following hair removal, reaching peak inflammatory response within 3-5 days. The condition may persist for weeks without intervention, particularly in individuals with coarse or curly hair textures that predispose to follicular penetration.
Herpetic lesions demonstrate a more predictable temporal pattern, with prodromal symptoms preceding visible lesions by 12-24 hours. The acute vesicular stage typically lasts 1-2 days, followed by ulceration and subsequent crusting over the following week. Recurrent episodes often exhibit shortened duration and reduced severity compared to primary infections, reflecting established immune recognition of viral antigens.
Pathophysiological mechanisms behind hair follicle occlusion
Understanding the underlying pathophysiology of follicular inflammation provides essential insights for accurate diagnosis and targeted therapeutic intervention. The development of ingrown hairs involves complex interactions between hair shaft morphology, follicular architecture, and mechanical trauma that culminate in inflammatory responses.
Keratinocyte hyperproliferation and comedone formation
The initial pathophysiological event in ingrown hair development involves alterations in normal keratinocyte turnover within the follicular infundibulum. Mechanical trauma from shaving or aggressive hair removal procedures stimulates excessive keratinocyte proliferation, leading to follicular hyperkeratosis and subsequent occlusion of the follicular opening. This process mirrors comedone formation in acne vulgaris, creating an environment conducive to bacterial colonisation and inflammatory responses.
The hyperproliferative response results in thickening of the follicular epithelium, which narrows the follicular canal and impedes normal hair shaft emergence. Simultaneously, increased desquamation of corneocytes within the follicle creates a protein-rich debris that further contributes to follicular obstruction. These combined factors create optimal conditions for hair shaft misdirection and subsequent inflammatory cascade activation.
Propionibacterium acnes colonisation in occluded follicles
Follicular occlusion creates an anaerobic microenvironment that favours the proliferation of Propionibacterium acnes , a commensal organism that becomes pathogenic under specific conditions. The bacteria metabolise sebaceous lipids, producing inflammatory mediators including free fatty acids and chemotactic factors that attract neutrophils and macrophages to the affected follicle.
This bacterial colonisation triggers both innate and adaptive immune responses, resulting in the characteristic inflammatory infiltrate observed in folliculitis barbae. The persistence of bacterial antigens within the occluded follicle maintains chronic inflammation, explaining the prolonged clinical course often observed in untreated ingrown hairs. Antibiotic therapy targeting these organisms often provides significant clinical improvement, supporting their pathogenic role in disease progression.
Mechanical trauma from curly hair penetration
The morphological characteristics of individual hair shafts significantly influence susceptibility to ingrown hair development. Curved or coiled hair structures demonstrate increased propensity for follicular re-entry, particularly following close shaving that creates sharp, angled hair tips. The mechanical penetration of these modified hair shafts through the follicular wall or adjacent skin creates micro-trauma that initiates inflammatory responses.
Biomechanical studies have demonstrated that hair shafts with increased curvature generate greater lateral forces during growth, increasing the likelihood of extra-follicular penetration. This mechanical trauma activates local inflammatory pathways, including complement cascade activation and cytokine release, culminating in the clinical manifestations of folliculitis barbae. Understanding these mechanical factors has led to targeted prevention strategies focusing on hair shaft modification and growth pattern alteration.
Sebaceous gland dysfunction and lipid composition changes
Sebaceous gland activity plays a crucial role in follicular health and susceptibility to inflammatory conditions. Alterations in sebaceous lipid composition, particularly changes in the ratio of saturated to unsaturated fatty acids, can influence follicular microenvironment and bacterial colonisation patterns. These changes often result from hormonal fluctuations, topical treatments, or genetic predisposition.
The modified lipid environment affects both the physical properties of sebum and its antimicrobial activity. Reduced antimicrobial efficacy allows increased bacterial proliferation, whilst altered sebum viscosity may contribute to follicular occlusion. These factors create a self-perpetuating cycle of inflammation that explains the chronic nature of folliculitis barbae in susceptible individuals. Therapeutic interventions targeting sebaceous gland function often demonstrate significant clinical benefit in managing recurrent cases.
Herpes simplex virus replication cycle and cutaneous manifestations
The pathophysiology of herpes simplex virus infection involves complex viral-host interactions that determine both acute manifestations and long-term clinical outcomes. Understanding these mechanisms provides crucial insights for differential diagnosis and therapeutic decision-making.
Viral DNA integration and neuronal latency establishment
Following initial infection, herpes simplex virus demonstrates remarkable ability to establish latent infection within sensory ganglia, creating lifelong potential for viral reactivation. The viral DNA persists in an episomal form within neuronal nuclei, remaining transcriptionally silent during latency periods. This unique characteristic explains the recurrent nature of herpetic lesions and their tendency to occur in consistent anatomical distributions.
The establishment of latency involves sophisticated viral mechanisms that evade host immune surveillance whilst maintaining genetic material in a replication-competent state. Periodic viral reactivation occurs in response to various triggers including stress, immunosuppression, or local tissue trauma. Understanding this cycle is essential for patient education regarding transmission risks and long-term management strategies.
Cytopathic effects on keratinocyte cell membranes
Viral replication within infected keratinocytes produces characteristic cytopathic effects that create the distinctive clinical appearance of herpetic lesions. The virus disrupts normal cellular metabolism and membrane integrity, leading to cell swelling, multinucleation, and eventual cell death. These changes result in the formation of intraepidermal vesicles that appear as the characteristic fluid-filled blisters.
The viral cytopathic effects also trigger local inflammatory responses that contribute to the erythematous base surrounding herpetic lesions. The combination of direct viral damage and inflammatory responses creates the clinical syndrome observed during active outbreaks. Antiviral therapy targeting viral replication can significantly reduce both the severity and duration of these manifestations when initiated during early stages of outbreak development.
Immunological response to HSV-1 glycoprotein antigens
The host immune response to herpes simplex virus involves both cellular and humoral components that influence clinical presentation and disease progression. Initial infection typically triggers robust inflammatory responses as the immune system recognises viral glycoproteins presented on infected cell surfaces. This primary immune response often results in more severe and prolonged symptoms compared to recurrent episodes.
Subsequent viral reactivations encounter established immune memory responses that typically limit viral replication and reduce symptom severity. However, the virus has evolved mechanisms to partially evade immune recognition, explaining the ability to cause recurrent infections despite established immunity. The balance between viral replication and immune control determines the clinical severity and frequency of outbreaks in individual patients.
Prodromal symptoms and viral shedding phases
The prodromal phase of herpes simplex virus reactivation provides important diagnostic clues that distinguish viral infection from mechanical follicular trauma. These early symptoms typically include localised tingling, burning, or pain that precedes visible lesions by 12-24 hours. The prodromal period corresponds to initial viral replication and migration along sensory nerve pathways to the skin surface.
Viral shedding begins during the prodromal phase and continues throughout the active outbreak period, representing the time of highest transmission risk. Understanding these phases is crucial for patient counselling regarding transmission prevention and optimal timing of antiviral therapy. Early intervention during the prodromal phase can significantly reduce outbreak severity and duration, making recognition of these early symptoms clinically important.
Diagnostic methodologies for differential identification
Accurate differentiation between ingrown hairs and herpes lesions often requires objective diagnostic testing, particularly in cases where clinical presentation remains ambiguous. Modern diagnostic techniques provide reliable methods for definitive identification, enabling appropriate therapeutic decisions and patient counselling.
Tzanck smear cytological examination techniques
The Tzanck smear represents a rapid, cost-effective diagnostic method for identifying viral cytopathic effects characteristic of herpes simplex infection. The procedure involves collecting cellular material from the base of suspected viral lesions and examining the specimen for multinucleated giant cells and other viral-induced morphological changes. Whilst not specific for herpes simplex virus, the presence of these characteristic cellular changes strongly supports viral aetiology.
The technique requires careful specimen collection from active lesions, preferably during the early vesicular stage when viral replication is most active. Proper staining and microscopic examination can provide results within minutes, making it particularly valuable in clinical settings where rapid diagnosis is essential. However, the sensitivity of Tzanck smears decreases significantly during later stages of outbreak when viral replication has diminished.
Pcr-based viral detection and genotyping protocols
Polymerase chain reaction technology provides the most sensitive and specific method for herpes simplex virus detection and typing. Modern PCR assays can detect viral DNA even when present in very low concentrations, making them particularly valuable for diagnosing atypical presentations or asymptomatic viral shedding. Additionally, these techniques can differentiate between HSV-1 and HSV-2, providing important information for prognosis and counselling.
Real-time PCR protocols offer rapid turnaround times, often providing results within hours of specimen collection. The high sensitivity of these assays allows detection of viral DNA from various specimen types, including vesicle fluid, swabs from ulcerated lesions, or even saliva samples in cases of oral infection. Genotyping capabilities provide additional clinical value by helping predict recurrence patterns and transmission risks.
Direct fluorescent antibody staining methods
Direct fluorescent antibody staining offers another approach for rapid viral diagnosis, utilising fluorescent-labelled antibodies specific for herpes simplex virus antigens. This technique can provide results within hours and offers good specificity for viral identification. The method requires fresh specimens and appropriate microscopy equipment, but can be particularly valuable in settings where PCR testing is not readily available.
The technique involves treating collected cellular material with fluorescent antibodies that bind specifically to viral proteins, creating visible fluorescence when examined under appropriate microscopy conditions. Positive results appear as bright green fluorescence within infected cells, providing clear evidence of viral infection. Proper specimen handling and processing are crucial for optimal test performance and accurate interpretation.
Bacterial culture and antibiotic sensitivity testing
When bacterial infection is suspected in cases of folliculitis barbae, bacterial culture and sensitivity testing provide valuable guidance for targeted antibiotic therapy. Specimens collected from pustular lesions can identify specific bacterial pathogens and determine their antibiotic susceptibility patterns, enabling selection of optimal therapeutic agents.
Standard culture techniques typically require 24-48 hours for organism identification and an additional 24 hours for sensitivity testing. However, this information proves invaluable for managing complicated cases or infections that fail to respond to empirical antibiotic therapy. The identification of specific organisms and their resistance patterns guides therapeutic decisions and helps prevent the development of antibiotic resistance.
Dermoscopic pattern recognition and follicular architecture
Dermoscopy provides a non-invasive method for examining lesion morphology and identifying characteristic patterns that aid in differential diagnosis. Ingrown hairs often display distinctive dermoscopic features including visible hair shafts beneath the skin surface, follicular plugging, and characteristic inflammatory patterns surrounding affected follicles.
In contrast, herpetic lesions typically lack these follicular-centred features and instead demonstrate vesicular structures, erosions, and healing patterns consistent with viral infection. Experienced practitioners can often achieve accurate diagnosis through dermoscopic examination alone, making this technique particularly valuable in clinical practice where rapid assessment is required.
Therapeutic intervention strategies and pharmacological approaches
The therapeutic management of ingrown hairs and herpes lesions requires distinctly different approaches, emphasising the critical importance of accurate diagnosis. Treatment strategies must address both acute symptom relief and long-term prevention of recurrent episodes, whilst considering individual patient factors and potential complications.
For folliculitis barbae and ingrown hair management, topical and systemic approaches target the inflammatory cascade and bacterial colonisation that perpetuate the condition. Topical retinoids such as tretinoin or adapalene
promote follicular exfoliation and reduce keratinocyte hyperproliferation within affected hair follicles. These agents work by normalising the keratinisation process and preventing follicular occlusion that predisposes to ingrown hair development.
Topical antibiotics including clindamycin or erythromycin solutions provide targeted treatment for bacterial colonisation within inflamed follicles. These preparations reduce Propionibacterium acnes populations whilst minimising systemic antibiotic exposure and associated resistance development. For more severe cases involving extensive inflammation or secondary bacterial infection, systemic antibiotics such as doxycycline or minocycline may be indicated.
Anti-inflammatory agents including topical corticosteroids can provide symptomatic relief during acute inflammatory phases, though prolonged use should be avoided due to potential follicular atrophy and skin thinning. Alternatively, topical calcineurin inhibitors such as tacrolimus offer anti-inflammatory benefits without the adverse effects associated with long-term corticosteroid application.
Herpes simplex virus management centres on antiviral therapy that inhibits viral replication and reduces outbreak duration and severity. First-line antiviral agents including aciclovir, valaciclovir, and famciclovir demonstrate proven efficacy when initiated during prodromal phases or early outbreak stages. These medications work by inhibiting viral DNA polymerase, effectively blocking viral replication cycles.
Topical antiviral preparations such as aciclovir cream or penciclovir cream provide localised treatment options for mild outbreaks, particularly in immunocompetent individuals. However, systemic antiviral therapy generally demonstrates superior efficacy and is preferred for moderate to severe outbreaks or in immunocompromised patients. Suppressive therapy with daily antiviral medication may be indicated for individuals experiencing frequent recurrent episodes.
Prevention protocols and long-term management considerations
Effective prevention strategies for both conditions require understanding of predisposing factors and implementation of targeted interventions that address underlying pathophysiological mechanisms. For ingrown hair prevention, modification of hair removal techniques represents the most critical intervention, with specific focus on reducing mechanical trauma and follicular disruption.
Pre-shaving preparation involving warm water application and use of appropriate lubricating agents helps soften hair shafts and reduce cutting resistance. Sharp razor blades maintained in optimal condition prevent hair shaft crushing and irregular cutting patterns that predispose to follicular re-entry. Shaving technique modification including single-pass shaving in the direction of hair growth minimises mechanical trauma whilst achieving acceptable cosmetic results.
Post-shaving care incorporating gentle exfoliation and moisturisation helps maintain healthy follicular architecture whilst preventing hyperkeratosis development. Chemical exfoliants containing salicylic acid or glycolic acid prove particularly effective at preventing follicular occlusion whilst reducing inflammation associated with minor trauma. Regular application of these agents can significantly reduce ingrown hair incidence in susceptible individuals.
For individuals with recurrent folliculitis barbae, alternative hair removal methods including laser hair removal or intense pulsed light therapy may provide long-term solutions by reducing hair density and modifying growth patterns. These treatments target melanin within hair follicles, causing selective thermal damage that reduces future hair growth and associated inflammatory responses.
Herpes simplex virus prevention focuses primarily on reducing transmission risks and identifying personal outbreak triggers that can be modified or avoided. Consistent use of barrier protection during sexual activity significantly reduces transmission risks, though complete prevention requires avoiding skin-to-skin contact during symptomatic periods when viral shedding is highest.
Trigger identification and modification represent crucial components of long-term herpes management. Common triggers including stress, fatigue, immunosuppression, and local trauma can often be identified through careful history-taking and outbreak pattern analysis. Stress management techniques including regular exercise, adequate sleep, and psychological support can significantly reduce outbreak frequency in stress-sensitive individuals.
Nutritional support including adequate protein intake and specific micronutrients such as zinc and vitamin C may support immune function and reduce outbreak severity. Some individuals benefit from lysine supplementation, though scientific evidence for this intervention remains limited. Regular medical follow-up ensures optimal management strategies and allows for treatment modification based on individual response patterns.
Long-term considerations for both conditions include monitoring for complications and ensuring appropriate patient education regarding symptom recognition and management strategies. Individuals with recurrent ingrown hairs should be counselled regarding proper hygiene practices and early intervention techniques that can prevent progression to more severe inflammatory responses. Similarly, herpes patients require education regarding transmission risks, symptom recognition, and the importance of early antiviral intervention.
The psychological impact of both conditions should not be underestimated, particularly for herpes patients who may experience significant emotional distress related to diagnosis and social stigma. Comprehensive management approaches must address both physical symptoms and psychological well-being through appropriate counselling and support services. Regular dermatological follow-up ensures optimal outcomes whilst monitoring for potential complications or treatment resistance.